The Atom
An atom is mostly empty space. Each atom has a very dense positive core called the nucleus that contains protons and neutrons. Electrons exist outside the nucleus in regions descirbed as electron clouds. If an atom were as big as a football field, the nucleus would be a dime on the 50-yard line and the electrons would be mosquitos on the sidelines.
- All matter is made of tiny particles called atoms.
- Atoms themselves are made of even smaller particles, called sub-atomic particles (electrons, protons, and neutrons)
- Even some of the sub-atomic particles are made of smaller things.
- There are about 100 different types of atoms, arranged into a periodic table of the elements.
- Although each type of atom differs because of the number and arrangement of the sub-atomic particles, all atoms have the same basic structure:
- A very small, positively charged nucleus that holds protons and neutrons. The nucleus is more massive but much smaller than the electrons.
- Electrons exist outside the nucleus in an “electron cloud” that is very large compared to the nucleus. Electrons are much less massive than the nucleons (protons and neutrons).
| Mass | |||
| Particle | kg | u | MeV/c2 |
| Electron | 9.1094×10−31 | 0.00054858 | 0.51100 |
| Proton | 1.67262×10−27 | 1.007276 | 938.27 |
| Hydrogen Atom | 1.67353×10−27 | 1.007825 | 938.78 |
| Neutron | 1.67493×10−27 | 1.008665 | 939.57 |
In the table above, the term rest mass refers to the mass of the particle when it is at rest. The actual mass changes as the speed of the particle gets really fast, however, don't worry about this until you have studied relativity.
The table above introduces two additional units for measuring mass, u (sometimes written as amu), and 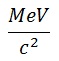 .
.
- The atomic mass unit, u is the mass of 1/12 of a Carbon-12 atom (i.e. a Carbon-12 atom has a mass of 12u). 1u = 1.66×10−27 kg
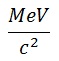 stands for Mega-ElectronVolt per speed of light squared. 1MeV/c2= 1.78×10−30 kg
stands for Mega-ElectronVolt per speed of light squared. 1MeV/c2= 1.78×10−30 kg- And finally, 931.5 MeV/c2 = 1u
Example - Sulfur at rest
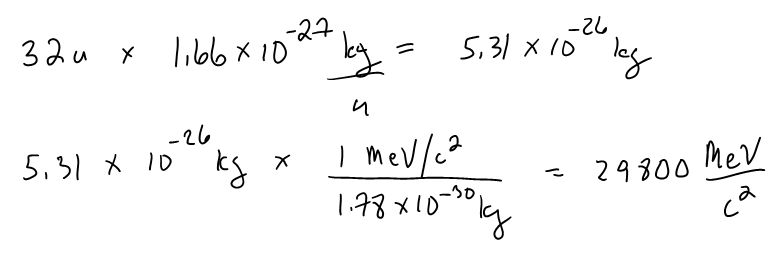
Using the Periodic Table to Model Atomic Structure
From any square on the periodic table, you should be able to determine the structure of that atom. Consider the element Uranium, number 98 on the periodic table.
download a periodic table here
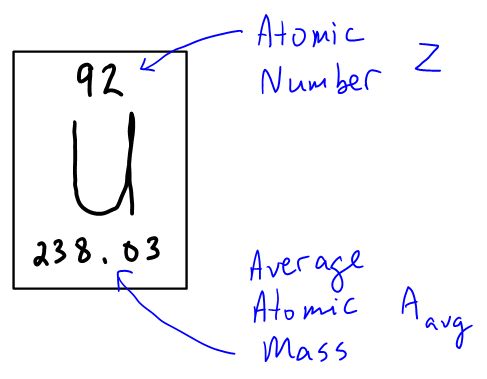
- The atomic number, Z, represents the number of protons in the nucleus of the atom. For neutral atoms (where + charge equals − charge) this is also the number of electrons.
- The average atomic mass, Aavg, represents, on average, the sum of the protons and neutrons that are in the nucleus of a typical example of this type of atom. Round this value to the nearest 1's place.
- A − Z equals the number of neutrons in the nucleus.
So the average uranium atom has 92 protons, 92 electrons and 146 neutrons.
Isotopes
It is the number of protons that determines the identity of an element. The periodic table is organized from left to right by the atomic number (number of protons). All uranium atoms, for example, have 92 protons. If it were to lose a proton, it would no longer be uranium, but would become protactinium.
However, an atom can change the number of other subatomic particles it has without losing its identity.
Atoms of the same type but with different numbers of neutrons are known as isotopes. Two of the isotopes for uranium have mass numbers of 238 and 235. We could write these isotopes in the following ways . . .
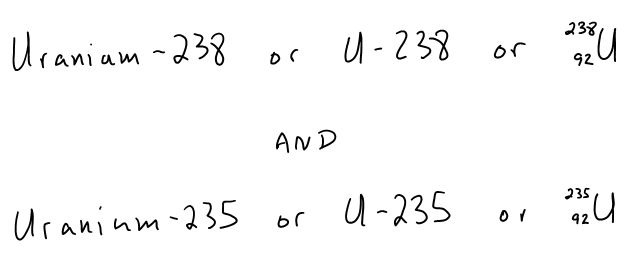
The average atomic mass (found on the periodic table) gives you a clue as to the most common isotope of that element. In any sample of an element there are billions of atoms, and all isotopes of that atom are present. Rounding the average atomic mass to the nearest 1's place will tell you which isotope is the most common.
Example - Desconstructing Atoms
protons - 83
electrons - 83
neutrons = 209 - 83 = 126